Layers of potential
Graphene, with its diversity of interesting properties, has been heralded as technology’s next big thing. But, nearly a decade after its discovery, are bendy phones all this material can offer?
Graphene, with its diversity of interesting properties, has been heralded as technology’s next big thing. But, nearly a decade after its discovery, are bendy phones all this material can offer?
For graphene
Researchers at the Massachusetts Institute of Technology (MIT) have recently announced the discovery of yet another fascinating property of graphene. The carbon-based material generates a large number of electrons when it absorbs light: making it a viable material with which to manufacture photovoltaic cells.
When researchers at the University of Manchester tried isolating graphene in 2004, it was thought to be too unstable to work with. Not only were they successful in isolating the material, it turned out to be so useful and fascinating that the researchers (Andre Geim and Konstantin Novoselov) were awarded the Nobel Prize for Physics in 2010.
Outstanding mechanical, optical, thermal and physical properties make this material very promising indeed
Graphene is only one atom thick, but it possesses a variety of intriguing properties. The carbon atoms are arranged in a honeycomb lattice, in a single-atom layer, which makes it a great electric conductor. Its outstanding mechanical, optical, thermal and physical properties make this material very promising indeed.
Though research is still underway, graphene’s remarkable diversity of properties means it is an attractive material for flexible electronics. Byung Hee Hong and Jong-Hyun Ahn, researchers at SKKU Advanced Institute of Nanotechnology, have already started experimenting with stacking layers of graphene to produce a film “with properties superior to those of commercial transparent electrodes such as indium tin oxides”.
Because the material absorbs only 2.3 percent of the light that hits it, and conducts electricity easily, by sandwiching a layer of liquid crystals between two graphene sheets, it becomes a ‘smart’ LCD screen (as light travels between the electrodes). Graphene has been used as a ‘Hall-bar device’ for sensitive electronic detection, but graphene’s photovoltaic capabilities are probably its most commercially appealing property.
Conventional materials with similar properties usually turn light into electricity at the rate of one electron per photon absorbed. A photon, however, has more energy than an electron is capable of carrying, so a lot of the light energy is lost as heat. Graphene appears to be able to generate several electrons per photon.
Graphene has another advantage over other photovoltaic materials like silicon and gallium arsenide; its optical properties mean it is a unique conductor. “Graphene can work with every possible wavelength you can think of”, explains Andrea Ferrari, Professor of Nanotechnology at the University of Cambridge. “There is no other material in the world with this behaviour.” This is exciting news for the solar energy industry, though it might still be a while before graphene photovoltaic panels come to be.
The research community is certainly very excited about graphene. As well as the Nobel Physics Prize won by Geim and Novoselov in 2010, and the research being carried out at MIT, a team led by Jari Kinaret from Chalmer’s University in Sweden has just been awarded €1bn by the EU to further their research. With that sort of backing, graphene will be a commercially viable material in no time.
Against graphene
Since its discovery by Konstantin Novoselov, Andre Geim and other researchers at the University of Manchester in 2004, much excitement has been stirring in the science world as talk of the potential uses of graphene grows. Many have heralded this tiny, one-atom-thick layer of carbon as the next big technological step forward, with uses as diverse as flexible and transparent electronics, protective coatings, solar cells, architectural designs and advanced transistors.
The resilience that comes with a material made from a hexagonal lattice of carbon atoms means designers have been desperate to introduce graphene as a commercially viable material in the production of a range of items. However, realising this dream has proven harder than expected. Despite the material being discovered almost a decade ago, we are yet to see any mainstream products made from it.
That it has been almost a decade without any real headway means much more research is needed
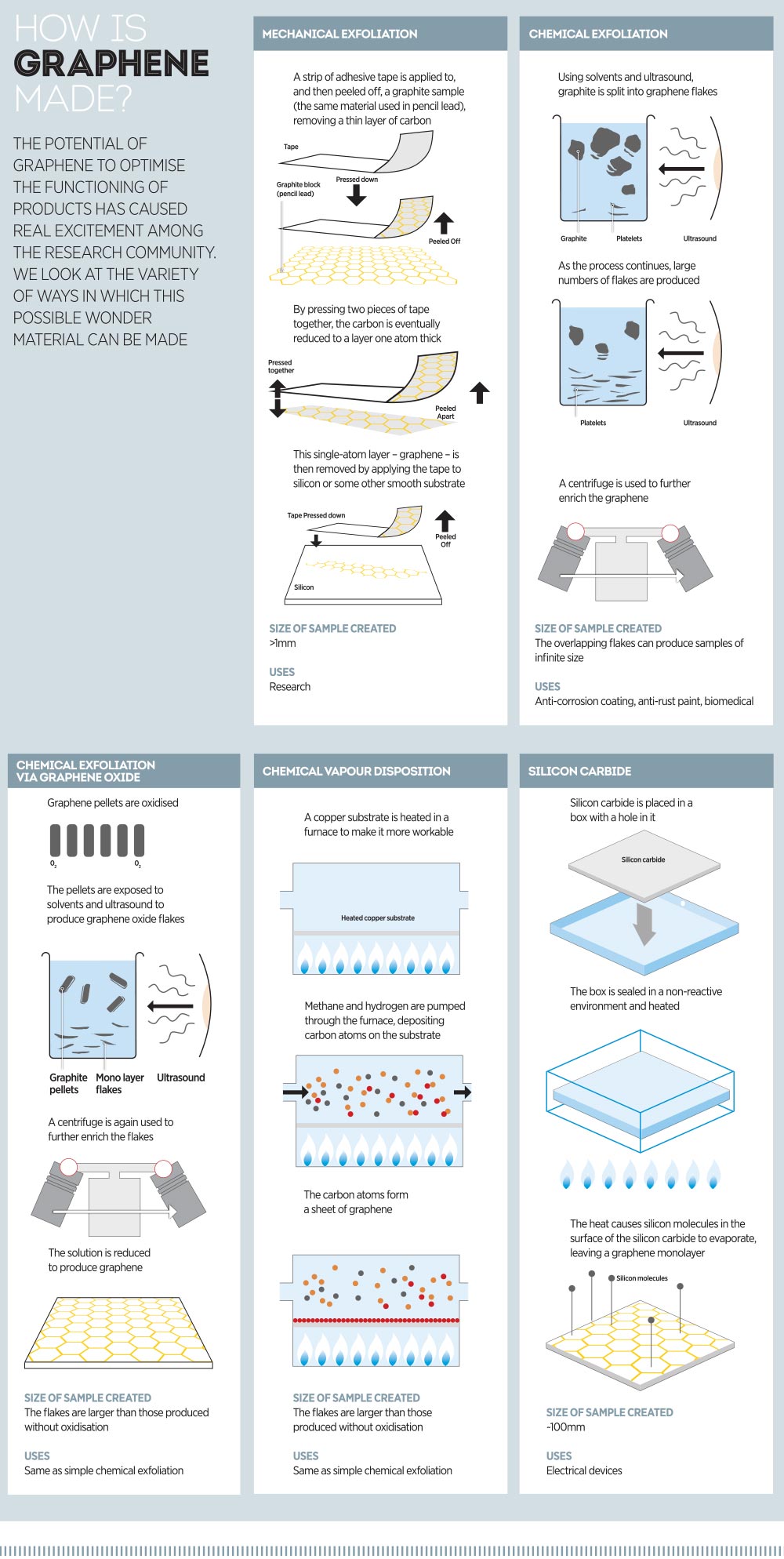
Much of the research seen so far has centred on mechanical exfoliation as a means of achieving the best forms of graphene, but it is both costly and time-consuming. For large-scale manufacturing, it is simply not yet practical. There is also concern that graphene transistors are not ready for production, mostly because they lack a band gap – vital in passing an electric current through a solid object – and a solution is not expected for another decade or so.
Even the material’s founders have recently written about the challenges facing the development of graphene as a widely used technology: specifically, whether the advantages it brings offer enough benefits to replace currently used materials.
Novoselov and other members of his research team published a paper titled “A Roadmap for Graphene” last October, spelling out their concerns about the commercial viability of the material. They conclude that, instead of graphene replacing other materials already actively in use in product design, it should be used in applications specifically designed to benefit from its unique properties.
Novoselov said graphene has the “potential to revolutionise many aspects of our lives simultaneously,” but that, while some products might be ready within a few years, depending on the quality of graphene required, “Different applications require different grades of graphene and those which use the lowest grade will be the first to appear, probably as soon as in a few years. Those which require the highest quality may well take decades.”
The current range of applications for graphene seems rather narrow. According to Novoselov and his team: “Graphene is a unique crystal in a sense that it has singlehandedly usurped quite a number of superior properties: from mechanical to electronic. This suggests that its full power will only be realised in novel applications, which are designed specifically with this material in mind, rather than when it is called [upon] to substitute other materials in existing applications.”
Graphene is clearly a remarkable discovery that should, in time, transform the design of a number of different products. However, the fact that it has been almost a decade without any real headway made means much more research is needed in bringing down the cost and improving the stability of this tiny piece of carbon.