Fighting cancer with innovative immunotherapy techniques
David J Mazzo of NeoStem explains how the company is teaching patients’ immune systems to recognise the cancer or tumour-initiating cells found in their specific tumours
Often the body's immune system does not recognise cancer cells as dangerous, as they are internal and not foreign to the body, which allows tumours to grow. Immunotherapy treatments aim to help the body recognise these threats
To understand how cancer immunotherapies work, it is useful to understand cancer’s defining properties. Cancer occurs when normal cells in the body multiply at an unusual rate and old cells do not die. Cancer also has the ability to invade and damage normal tissue, either locally (close to the site of the initial tumour) or distantly (at other sites throughout the body). A healthy immune system may have trouble fighting cancer, first because cancer cells are internal and not foreign to the body, so the immune system might not recognise them as dangerous, and, second, because some cancer actually suppresses immune activity.
NBS20 uses the patient’s own immune cells and tumour-initiating cells to create a therapeutic vaccine
Immunotherapies are treatments that fight disease using components of a patient’s own immune system – either by stimulating the patient’s immune system or by providing the patient with manmade products that stimulate the immune system. Cancer immunotherapies currently in development can be divided into a few major groups, such as monoclonal antibodies, checkpoint inhibitors and cancer vaccines.
Antigens are the molecules on viruses, bacteria or tumours that are recognised as foreign by the immune system. In a healthy person, the immune system develops proteins called antibodies that target these antigens, resulting in the destruction of the illness-causing microbes. Researchers have discovered how to make monoclonal antibodies that target particular antigens, including the antigens found on cancer cells, in laboratories. Some monoclonal antibodies (mAbs) currently available as cancer therapies include Bevacizumab (Avastin) for the treatment of colorectal, non-small cell lung and metastatic breast cancer, and Cetuximab (Erbitux) for the treatment of head and neck and colorectal cancer.
Scientists have discovered that one particular antigen, CTLA-4, prevents T cells (immune system cells that can destroy cancer cells) from doing their job, thereby making it easier for cancer to develop and spread unchecked. So researchers have developed an antibody in the lab called anti-CTLA-4 that blocks this ‘immune checkpoint’ antigen, freeing the immune system to attack and inactivate tumors. Anti-CTLA-4 is one example of what is known as a checkpoint inhibitor and has been approved by the US Food and Drug Administration (FDA) as ipilimumab (Yervoy) for the treatment of metastatic melanoma. Other checkpoint inhibitors react with programmed death 1 (PD1), which can inactivate cytotoxic T lymphocytes so they do not kill tumour cells they recognise. Nivolumab (Opdivo) and Pembrolizumab (Keytruda) have been approved for the treatment of metastatic melanoma.
Traditional preventive vaccines contain weakened versions of antigens that cause specific diseases, such as measles, mumps or flu. When injected into the body, the immune system can recognise and remember the antigen, so that, if a person becomes exposed later, the immune system will fight it, preventing illness. Therapeutic cancer vaccines are different in that they aim to treat, not prevent, cancer.
Other immunotherapies available include CAR-T therapy, which involves engineering immune T cells to help them recognise specific antigens on tumour cells, and TIL therapy, in which immune cells called tumour-infiltrating lymphocyte (TIL) cells are taken from the patient and the most effective cancer-killers among them are reproduced in a laboratory and then injected back into the patient.
Some immunotherapies are given in conjunction with other therapies, such as chemotherapy, while others are given afterwards, either to allow other therapies to weaken or shrink the cancer first, or as a second or third option if other treatments prove ineffective.
Helping the immune system
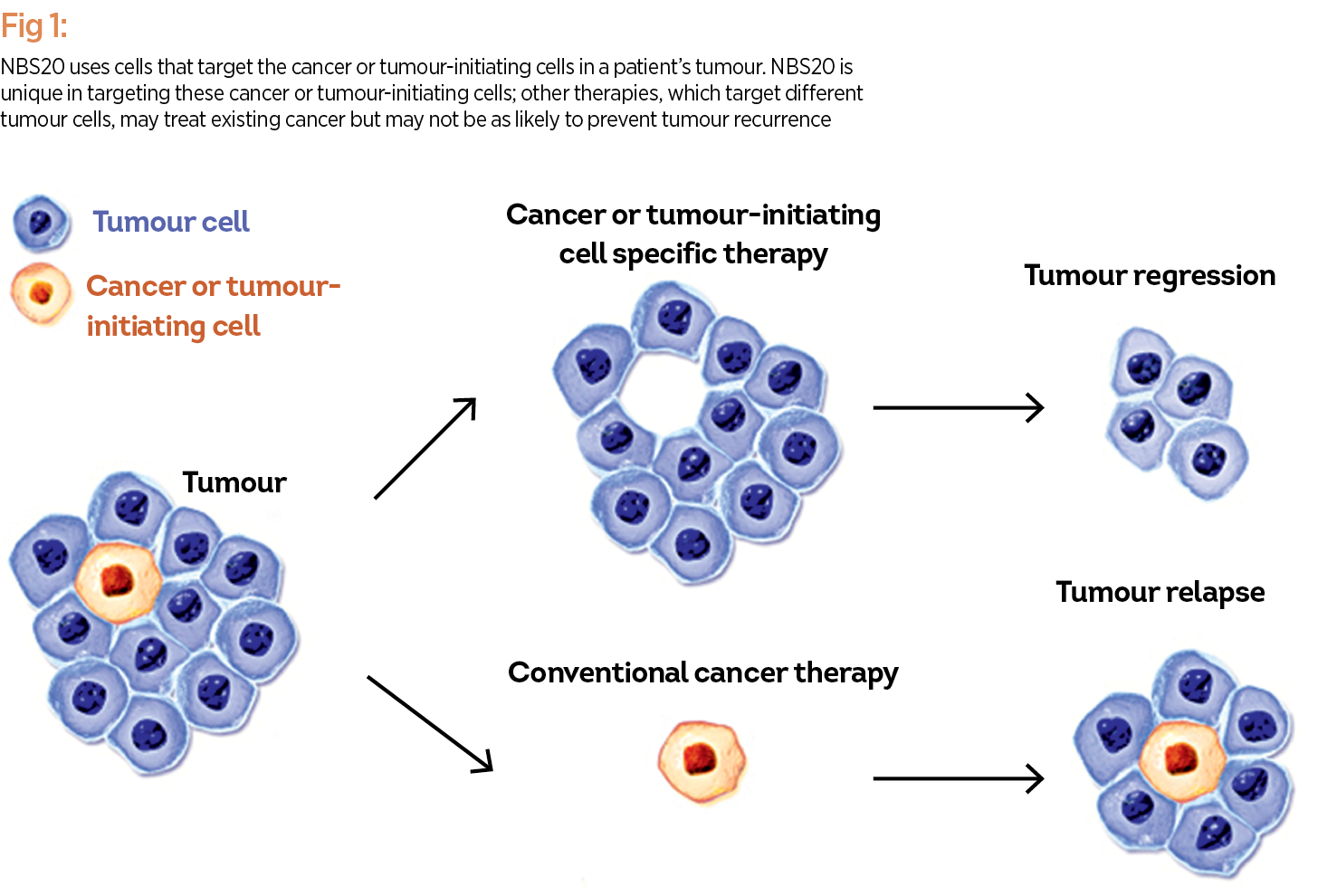
A patient-specific cancer immunotherapy, known as NBS20 (USAN generic name eltrapuldencel-T), is currently under late stage (Phase 3) clinical investigation by NeoStem. The proposed treatment is intended to help the immune system target cancer or tumour-initiating cells (commonly referred to as ‘cancer stem cells’), which are thought to rapidly proliferate cancer cells, fuelling tumour growth, and ultimately spreading the disease throughout the body. NBS20 uses the patient’s own immune cells and tumour-initiating cells to create a therapeutic vaccine. NBS20 is unique in targeting these cancer or tumour-initiating cells; other therapies, which target different tumour cells, may treat existing cancer but may not be as likely to prevent tumour recurrence (see Fig. 1).
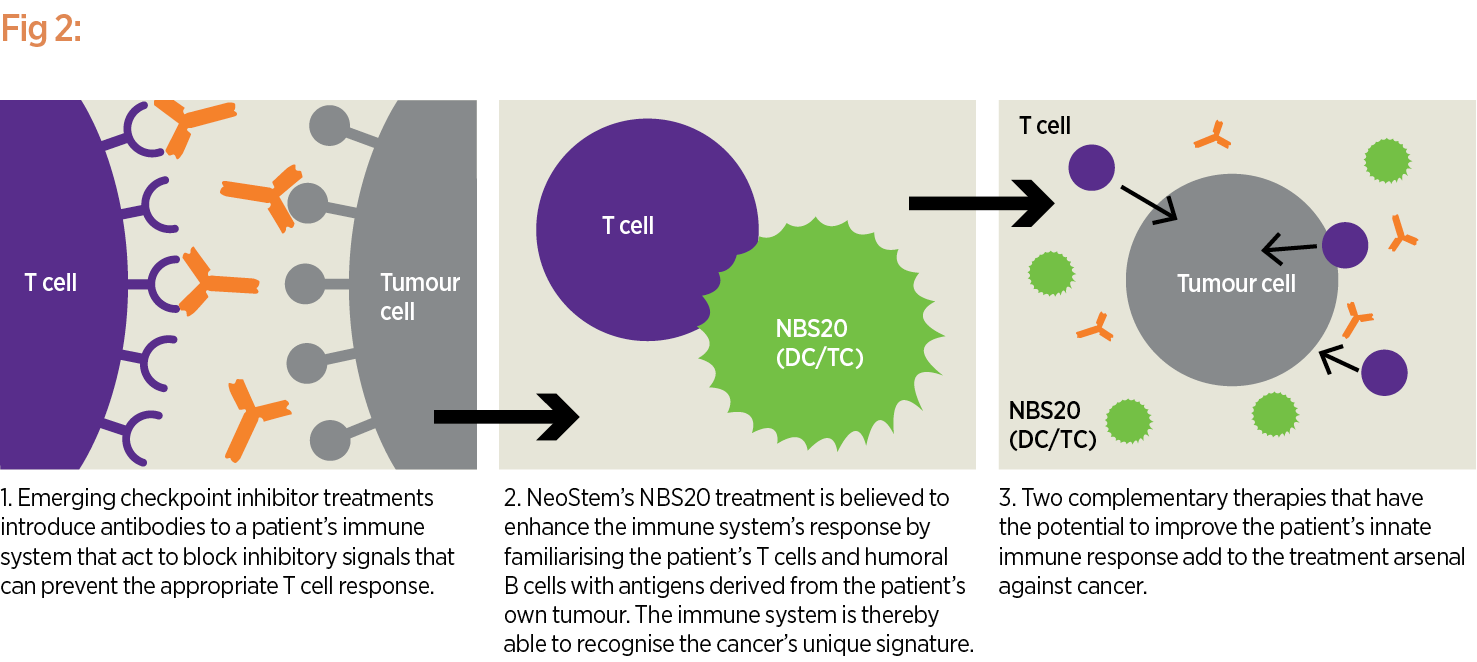
The goal is for NBS20 to eliminate these cancer or tumour-initiating cells after a patient has already undergone other treatments that may have reduced tumour size but are unlikely to result in a five-year survival (see Fig. 2). Results from a randomised Phase II trial, which showed a survival advantage for patients treated with DC-TC (now NBS20) compared to another investigational control treatment, were presented and published in 2012. Enrolment for the Phase III trial is taking place now at sites across the country.
Clinical efficacy and reasonable cost
Despite advancements in cancer immunotherapy, the cost of developing autologous treatments – those developed from a patient’s own cells – can be prohibitive. Maintaining the necessary clean room laboratory environments, employing skilled scientists, and manufacturing and producing each patient’s therapy individually, often through extensive manual production processes, can all lead to product cost of goods that is not sustainable through commercialisation, and therefore to treatments that may not be affordable for patients. To achieve success as an industry, manufacturers of cell therapies must be able to produce a high-quality product at a reasonable cost of goods, which also meets demand over the commercial life of the product.
NeoStem acquired PCT, a contract and development manufacturing organisation, in 2011. This acquisition provides NeoStem with significant cell therapy development expertise as well as cGMP (current good manufacturing practices) manufacturing facilities with controlled environments, thereby facilitating the clinical manufacturing of NeoStem’s own pipeline.
Through the application of PCT’s expertise and experience, NeoStem expects commercial-scale cost of goods to be manageable for its therapeutic candidates and for client product candidates, while still meeting FDA and cGMP standards. This is accomplished by implementing a structured manufacturing development methodology called Development by Design (DbD). Expanding on the foundational focus on quality of cell therapy products, DbD aims to help clients develop a commercially viable manufacturing process that focuses on four key drivers: quality, cost of goods, scalability, and sustainability.
An important step for cell therapy companies to achieve this is to define a product profile early in the development programme. Elements to consider in the profile include: characteristic profile (i.e. description, formulation, dosage, potency, volume and shelf life); safety profile (i.e. microbial assurance, cellular impurities and manufacturing residuals); use profile (i.e. indications for use, treatment timing, preparation and use); and business profile (i.e. geography market projections, cost of goods targets, and intellectual property).
Considering these factors as early in development as possible enables cell therapy companies to create a strategic development roadmap that anticipates challenges and manages the changes that will be required as development progresses. Based on the roadmap, we engage PCT’s deep scientific and engineering expertise and pursue innovation to overcome the challenges and meet the DbD goals for commercially viable manufacturing.
We believe our unique focus on targeting the tumour-initiating ‘stem’ cells within a tumour, combined with an innovative business model that consists of a rich cell therapy pipeline, externally recognised manufacturing expertise, and initiatives intended to use innovation to solve the challenges specific to the cell therapy industry, puts NeoStem in the unique position of simultaneously taking part in and shaping the future of cancer immunotherapy.













