Interview: printed prosthetics to disrupt healthcare industry
Streamlining costs and increasing provision are the two biggest hurdles facing the global healthcare industry. One firm believes 3D printing and additive manufacturing are the answers to both
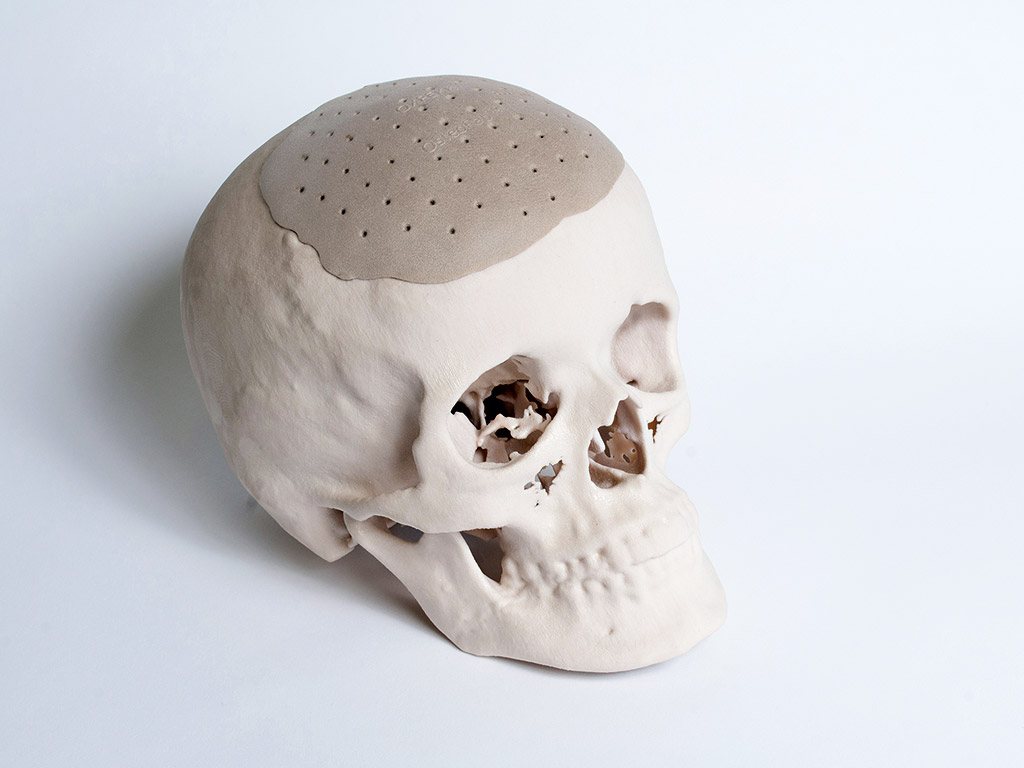
The OsteoFab Patient Specific Cranial Device is OPM’s first approved implant
As people continue to live longer and countries become more developed, the need for wider healthcare cover is becoming starker. The increase in costs associated with providing people with the health services they need has proved a challenge to governments and healthcare firms the world over.
While many expect 3D printing (or more correctly, ‘additive manufacturing’) to revolutionise a number of health-related industries, in particular prosthetics, there have been difficulties in finding the right materials that will get the best results from the technology. However, recent years have seen advances that may provide the industry with the disruptive technology to bring about this shift in price and provision.
The firm has long been an expert in providing a range of internal process technology with additives and formulations
One firm that has been leading the way in developing materials for prosthetics, while utilising advanced 3D printing technologies, is US-based Oxford Performance Materials (OPM). The firm has long been an expert in providing a range of internal process technology with additives and formulations prior to printing for a number of markets, most notably in the area of advanced materials for prosthetics. OPM has established itself as a leader in ‘high performance additive manufacturing’, delivering fully functional end-use products by focusing on the optimal material selection, process development, engineering expertise and product validation. The company was selected in 2012 as a key participant in the US government’s National Additive Manufacturing Innovation Institute.
OPM’s use of additive manufacturing offers a different proposal from normal 3D printing (which most frequently refers to rapid prototyping, the production of representations of functional products for planning and development purposes), and the company is developing a range of products that could revolutionise healthcare. OPM recently developed a prosthetic skull and ‘prints’ a range of cranial prostheses for implant each week. With the help of the technology, which received FDA clearance last year, it could prove groundbreaking for people with serious head injuries. OPM is also working towards FDA clearances for its OsteoFab devices designed for mid-face, spine and foot indications.
OPM’s CEO Scott DeFelice spoke to us about the potential of additive manufacturing for the healthcare industry, and why his company is “offering more for less”.
How has OPM grown over the years?
OPM was founded in 2000 as a materials company, working on one particular molecule called PEKK (polyetherketoneketone), which is a high performance thermoplastic. We traditionally sold materials into a number of different industries, such as biomedical, aerospace, semiconductors and energy, where we helped with the extraction of oil and gas. So we’ve always sold this high value material.
It was around 2005 that we started selling implantable materials. We did all of the heavy lifting necessary to establish biocompatibility and purity, and then began selling bars of the material for orthopaedic use, primarily for spine and spinal fusion. There’s a long history of this material being used in tens of thousands of surgical procedures, so this particular class of polymers has been known to be pure and compatible with the body.
From 2005 onward, we started to think about 3D printing of PEKK. We started talking to surgeons, and the consensus was that, if we could print our materials it would be highly beneficial. We ended up purchasing some highly advanced 3D printing equipment from EOS, which is a German manufacturer of laser sintering machines. We licensed some technologies from them so we could use our own materials in their equipment.
In March 2013, we got our first FDA implant clearance, which was the cranial prosthesis, called OsteoFab Patient Specific Cranial Device. We subsequently engaged market leader Biomet to handle global distribution on that product. At present, we’re manufacturing the cranial products for Europe, the US, the Middle East, South America, South Africa, and we’ve just filed for Japan.
How can 3D printing enhance the surgical and treatment options for patients?
With 3D printing, the potential to make a shape from a digital file has been around for 25 years. So what’s revolutionary is not that you have a process that can do that, it’s that you can have a process that utilises a material that has the utility to do that. The value chain for rapid prototyping has been led by machine suppliers that bundle hardware, software and materials. Bundling is possible in that area since the physical shape of the part typically drives product value (and not its mechanical performance or functionality). In contrast, OPM’s value chain is driven by many factors: the right material (or materials) needed to satisfy the desired product or part functionality combined with the process engineering necessary for production.
We call it ‘high performance additive manufacturing’ or ‘HPAM’ – we align specific materials development and engineering processes to efficiently ‘print useful things’. There’s no generic statement that covers 3D printing: there’s a multitude of 3D printing processes. When you look at those processes, some will never have the ability to print useful things, and some will: some of them are developing highly desirable economies of scale, and some of them aren’t. We have tried to focus on the particular space where we’re printing useful things and we enjoy highly desirable economics from the technology we’ve developed.
Are there any technological developments in medical 3D printing in the pipeline?
The near to medium term products we’re developing are for cranial, mid-face, spine, diabetic, and foot trauma products. For the medium to longer term, we’re focusing on drug eluting implants that can deliver therapeutics.
In the coming months, we will be announcing a cooperation with a world-renowned research university. We will be entering into joint development activity, including specific advances into that area of drug eluting implants. This is primarily to address implant related infections, and is all enabled by novel capabilities of additive manufacturing.
Isn’t 3D printing still relatively unaffordable for many patients? What can be done to challenge that?
The rising cost of healthcare is a global issue that cannot be ignored. In short, we need to provide healthcare to more people and do it with less money.
Our proposition is that the current materials and processes that have been used to solve healthcare problems in orthopaedics – which is our particular area of interest with 3D printing – have really run up against a wall in how far they can reduce costs and how far they can advance clinical efficacy. Our proposition is that going from metals to the PEKK polymer – which is more bone-like in composition and behaviour, less expensive, and easier to work with from a surgical point of view – is a disruptive change that will allow the industry to respond to those big trends.
We’ve already achieved our original goal of providing OsteoFab clinical solutions at equal or lower cost to current orthopedic implant options – ‘more for less’. And when you include the additional benefits our patient-specific devices are designed to deliver – reduced operating room time, reduced complications, less revision surgeries – the overall system costs savings and patient benefits are substantial.
What is exciting about this technology is that additional complexity does not significantly increase manufacturing cost. As a result, we believe that AM has the potential to provide improved healthcare in orthopaedics on a global scale – for developed and developing countries. This is important and necessary.
How do you see this technology spreading around the world?
I think that, within the next five years, you’re going to see a lot of product movement. Within the next 15 years, all major joints will be made this way – patient-specific, personalised medicine, where the end-use functional needs drive the composition and the process. The big markets, such as knees and hips, will be adopting this technology.
We are already seeing an evolution from standard ‘3D prototype’ machine offers to integrated, engineered ‘additive manufacturing solutions’. The latter are appropriate for the materials of construction and optimised for the volume, geometry and performance requirements of the specific end-use products. Ends do not justify means, but the additive manufacturing solution is based upon ends driving the means, and the materials and process development will be what define the modern additive manufacturing value chain.
Can you describe the technical convergence that has made 3D-printed personalised medical devices possible?
The equipment itself has been around for a while. The software that enables somebody to undertake sophisticated, controlled design and development got up to speed in the last five years. The computational capacity has been around for a decade. The final piece of the puzzle has really been the material sets that enable the whole thing.
That is where OPM is on the forefront. We’re an early entrant into selling these materials into critical applications. It’s easier for us to do this because we can just buy the right piece of equipment, and we already have extensive knowledge of the materials, processing and engineering required to generate a functional part. It’s been more than a decade-long journey and we’ve learnt an enormous amount along the way. We also listen to the market and apply science to the market: we don’t do it the other way round.













